Hector F. Rios
Department of Periodontics & Oral Medicine, School of Dentistry,
University of Michigan, Ann Arbor, MI, USA
Periodontal diseases are a possible modifiable risk factor for morbidity and mortality in several systemic diseases1-4 with an obvious impact in the patient’s quality of life5,6. Collectively, they afflict over 80% of adults worldwide and approximately 13% displaying severe disease concomitant with early tooth loss7. In periodontitis, the detrimental changes that the tooth-supporting tissues undergo are primarily the result of specific microbial challenges8-10. These challenges in a susceptible host disrupt the functional and structural integrity of the tooth supporting apparatus11. It is known that the structure and properties of the periodontal tissues are intimately related by crucial cell-matrix interactions12-16. However, the mechanisms by which these microorganisms undermine and compromise the modulation of these synergistic events are not clearly understood. The proper regulation of these interactions in a mechanically dynamic environment, such as in the periodontium, determines the adaptive dentalalveolar response by orchestrating the function of important bioactive proteins such as growth factors, cytokines and proteases (Figure 1)17-20. The proteins modulating these interactions are known collectively as matricellular molecules21-24. The purpose of this review is to present and discuss the available evidence that exist regarding the matricellular molecule Periostin in the context of the periodontium.
What is Periostin?
Periostin is a disulfide-linked 90 kDa secreted protein of relatively unknown function that has homology with the insect’s central nervous system protein fasciclin-I and is highly conserved among higher organisms25. The protein is highly homologous to βig-h3, a molecule induced by TGF-β that promotes the adhesion and spreading of fibroblasts. Therefore, Periostin is thought to function as an adhesion molecule during bone formation and can support osteoblastic cell line attachment and spreading26. Purified recombinant Periostin has been shown to be a ligand for αvβ3 and αvβ5 integrins, promoting integrin-dependent cell adhesion and motility27. Multiple reports have also demonstrated elevated Periostin levels in neuroblastoma28, epithelial ovarian cancer27 and in non-small cell lung carcinoma29 that have undergone epithelial mesenchymal transformation (EMT) and metastasized. Moreover, it has been shown that Periostin potently promotes cell survival via the Akt/PKB pathway30. It has also been associated with extracellular matrix (ECM) deposition following myocardial infarction and has been reported to be essential for healing after acute myocardial infarction31,32.
Why Periostin?
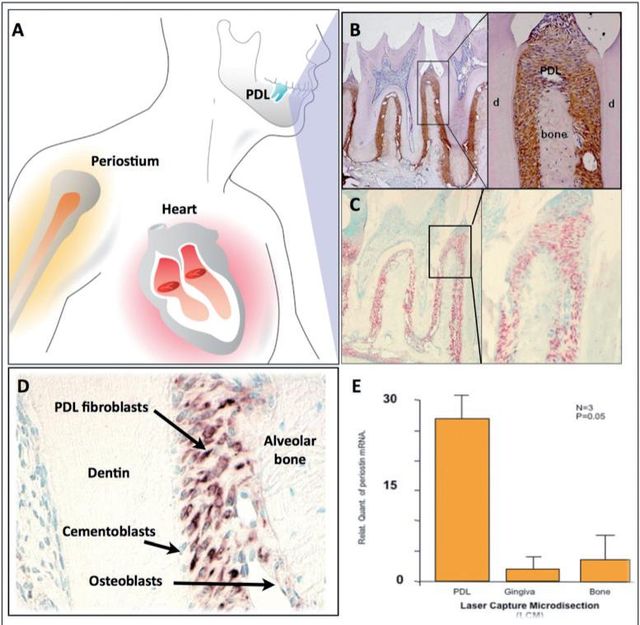
Previously, it has been shown that Periostin is expressed in the developing teeth at sites of epithelialmesenchymal interaction, which suggests that Periostin could play multiple roles as a primary responder molecule during tooth development and may be linked to deposition and organization of other ECM adhesion molecules during maintenance of the periodontium33. Periostin mRNA has been found to be negatively regulated by Epidermal Growth Factor and 1,25-(OH)2 D3 and upregulated by TGF-β1 and BMP- 225,26,34. Interestingly, immunohistochemical analysis has shown that, in adult mice, Periostin protein is preferentially localized in the periosteum and PDL26, suggesting potential roles in the maintenance and remodeling of these structures. Of all the known proteins that have been found to be expressed in the PDL, Periostin has been the one that shows greater specificity. For this reason, Periostin is commonly used as a marker for the PDL. Periostin, within the periodontal context, is expressed specifically by PDL fibroblasts, suggesting a potential role in PDL function26,33 (Figure 2). Recently, it was reported that Periostin promoter activities were enhanced by overexpression of Twist, resulting in increased Periostin expression in vitro35. In addition, in vivo, Twist and Periostin have been found to be co-expressed and intimately regulated by changes in the occlusal force36which led researchers to postulate Twist as a Periostin transcription factor. Interestingly, Periostin has shown to present a divergent distribution during orthodontic tooth movement; it is upregulated in the areas of compression and down regulated in the areas of tension37. Furthermore, immunoelectron microscopic observation of the mature PDL verified the localization of Periostin between the cytoplasmic processes of periodontal fibroblasts and cementoblasts and the adjacent collagen fibrils38. Its expression has been reported to influence cell behavior as well as collagen fibrillogenesis39. Therefore, it may exert control over the structural and functional properties of PDL tissues in health and disease.These findings suggest that Periostin is present at the sites of the cell-to-matrix interaction, serving as an adhesive factor for bearing mechanical forces, including occlusal force.
Is Periostin necessary for normal periodontal function?
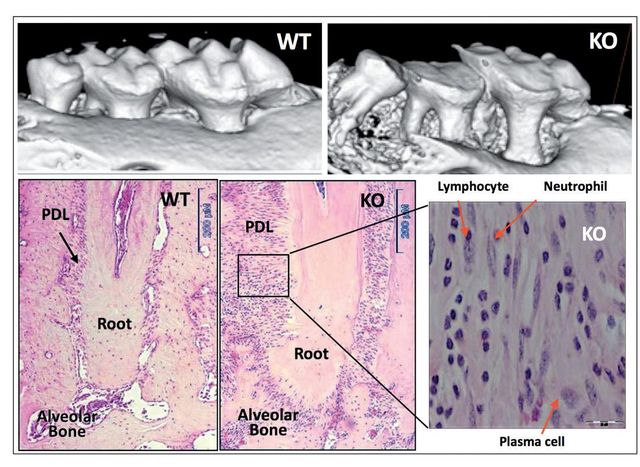
We provided initial evidence of the essential role of Periostin in the functional and structural integrity of the periodontium40. Periostin-deficient animals clearly show severe deterioration of the tooth supporting structures40,41. Our work demonstrated that in the context of Periostin deletion, the periodontium rapidly deteriorates over time and is unable to sustain the physiologic mechanical stimulus. Thereby, deeming this molecule as necessary for PDL function and, therefore, for proper periodontal integrity (Figure 3). Furthermore, we have shown that Periostin is specifically necessary during occlusal function, and that in the case of the Periostin KO mice, the periodontal ligament is unable to sustain the normal physiologic occlusal load, which results in a traumatic stimulus to the periodontium. While the literature suggests that primary occlusal trauma leads to an adaptive response where no destruction of the supporting tissues occurs, in the Periostin KO the mechanical stimulus can be classified as a secondary trauma that, due to the Periostin deficiency, causes a detrimental effect in the periodontium. This leads to severe alveolar bone loss, severe clinical attachment loss and significant widening of the PDL space.






[…] >English Version> […]